MARGENZA delivered more time without disease progression1
In the pivotal SOPHIA trial, MARGENZA plus chemotherapy demonstrated superior PFS compared with trastuzumab plus chemotherapy1,*
MARGENZA delivered a 24% reduction in risk of disease progression or death1
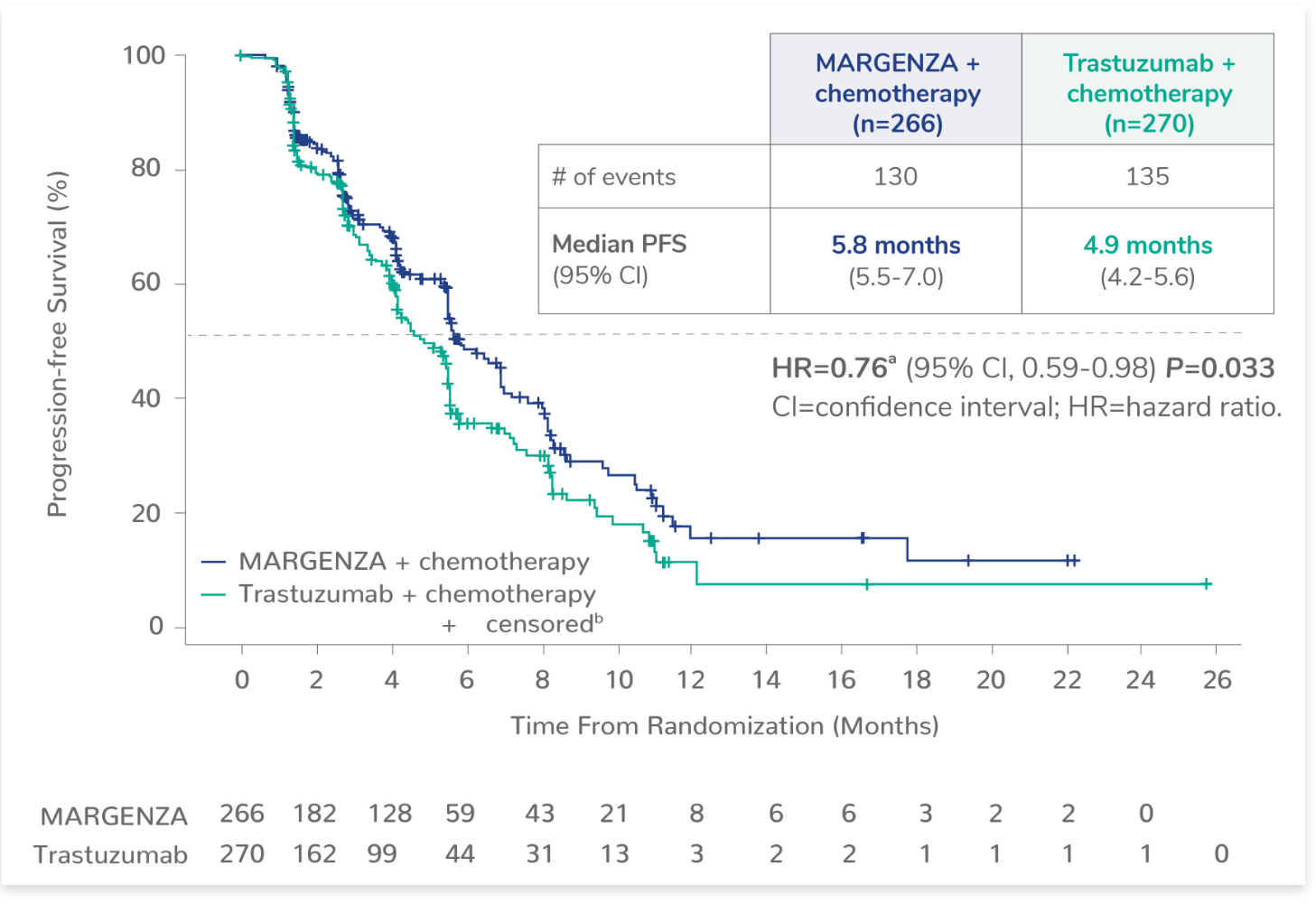
*PFS was evaluated by blinded independent central review (BICR).
aBased on stratified Cox Model.
b+ censored indicates censored data.
Explore the SOPHIA Study, including Study Design, the complete list of Premedications, and Safety Profile seen in the study.
Additional efficacy endpoints
|
MARGENZA + chemotherapy |
Trastuzumab + chemotherapy |
|
|---|---|---|
|
OS2,c
Median (months) (95% CI) |
(n=266) 21.6 (18.9-25.1) |
(n=270) 21.9 (18.7-24.2) |
|
Objective response rate for patients with measurable disease1,d
Confirmed ORR (%) (95% CI) |
(n=262) 22 (17-27) |
(n=262) 16 (12-20) |
|
Duration of response1
Median (months) (95% CI)e |
(n=58) 6.1 (4.1-9.1) |
(n=42) 6.0 (4.0-6.9) |
cHR=0.95 (95% CI, 0.77-1.17). The OS analysis for the intent-to-treat population did not demonstrate a statistically significant advantage.
dAssessed per BICR.
eBased on Kaplan-Meier estimates.
Explore prespecified exploratory subgroups by Fc𝛄 receptor genotype
See Exploratory PFS Analysis by CD16A Genotype
See Exploratory OS Analysis by CD16A Genotype
SOPHIA study design
The efficacy and safety of MARGENZA plus chemotherapy compared with trastuzumab plus chemotherapy was evaluated in SOPHIA, a randomized, multicenter, open-label trial of 536 metastatic HER2-positive breast cancer patients† who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
†IHC 3+ or ISH-amplified HER2+.

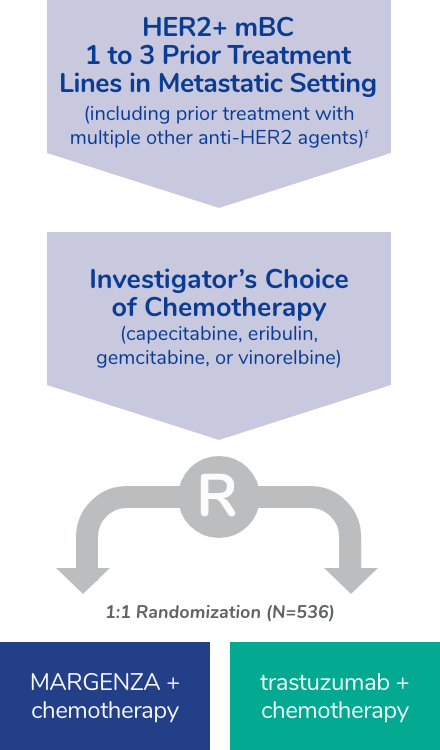
fThe median number of prior lines of therapy in the locally advanced/metastatic setting was 2 (range: 1-4). All study patients had previously received trastuzumab, all but 1 patient had previously received pertuzumab, and 91% had previously received ado-trastuzumab emtansine; 47% had visceral disease, 57% had bone metastases, 13% had brain metastases, and 60% were hormone receptor positive.
- The main efficacy outcome measures were PFS by BICR and OS. Additional efficacy outcome measures were ORR and DOR assessed by BICR
- Patients were randomized (1:1) to MARGENZA plus chemotherapy or trastuzumab plus chemotherapy. Randomization was stratified by chemotherapy choice (capecitabine, eribulin, gemcitabine, or vinorelbine), number of lines of therapy in the metastatic setting (≤2, >2), and number of metastatic sites (≤2, >2)
- Patients were required to have progressed on or after the most recent line of therapy. Prior radiotherapy and hormonal therapy were allowed
- Patients were treated with MARGENZA or trastuzumab in combination with chemotherapy until disease progression or unacceptable toxicity
- Patients received MARGENZA intravenously at a dose of 15 mg/kg every 3 weeks administered over 120 minutes for the initial administration and then over 30 to 120 minutes thereafter
Premedications in the SOPHIA Study
Premedications and prophylaxis for chemotherapy3
- In the SOPHIA trial, standard premedications for the administration of the cytotoxic chemotherapy were employed. For chemotherapy, recommendations made in the locally approved label should be followed for each agent
- When MARGENZA infusions coincided on the same day with cytotoxic chemotherapy administration, the same premedications for the chemotherapy were employed
- The following premedications were made available as per the SOPHIA study protocol and were given to a patient within 30 minutes prior to administering MARGENZA on those days upon which that administration was not preceded by a chemotherapeutic agent or if no premedication was employed for that chemotherapy
- Acetaminophen 650 mg-1000 mg orally (PO) or ibuprofen 400 mg PO
- Diphenhydramine 50 mg PO or IV or equivalent H1 antagonist
- Ranitidine* 300 mg PO or IV or equivalent H2 antagonist
- Dexamethasone 10 mg IV or equivalent (for patients at high risk)
*Effective April 1, 2020, the FDA requested manufacturers to withdraw all prescription and over-the-counter ranitidine drugs from market.
See dosage and administration information
Learn more about the
SOPHIA study
MARGENZA has a well-characterized safety profile
Safety of MARGENZA in combination with chemotherapy1
Adverse reactions (>10%) in patients with metastatic HER2+ breast cancer who received MARGENZA in SOPHIA
| Adverse Reaction | MARGENZA + chemotherapy (n=264) | Trastuzumab + chemotherapy (n=266) | ||
|---|---|---|---|---|
| All grades (%) | Grade 3 or 4 (%) | All grades (%) | Grade 3 or 4 (%) | |
| Fatigue/Asthenia | 57 | 7 | 47 | 4.5 |
| Nausea | 33 | 1.1 | 32 | 0.4 |
| Diarrhea | 25 | 2.3 | 25 | 2.3 |
| Vomiting | 21 | 0.8 | 14 | 1.5 |
| Constipation | 19 | 0.8 | 17 | 0.8 |
| Headacheg | 19 | 0 | 16 | 0 |
| Pyrexia | 19 | 0.4 | 14 | 0.4 |
| Alopecia | 18 | 0 | 15 | 0 |
| Abdominal painh | 17 | 1.5 | 21 | 1.5 |
| Peripheral neuropathyi | 16 | 1.1 | 15 | 2.3 |
| Arthralgia/Myalgia | 14 | 0.4 | 12 | 0.8 |
| Cough | 14 | 0.4 | 12 | 0 |
| Decreased appetite | 14 | 0.4 | 14 | 0.4 |
| Dyspnea | 13 | 1.1 | 11 | 2.3 |
| Infusion-related reaction | 13 | 1.5 | 3 | 0 |
| Palmar-plantar erythrodysesthesia | 13 | 0 | 15 | 3 |
| Extremity pain | 11 | 0.8 | 9 | 0 |
gIncludes headache and migraine.
hIncludes abdominal pain, abdominal discomfort, lower abdominal pain, and upper abdominal pain.
iIncludes peripheral neuropathy, peripheral sensory neuropathy, peripheral motor neuropathy, and neuropathy.
Serious adverse reactions occurred in 16% of patients who received MARGENZA.
- Serious adverse reactions in >1% of patients included febrile neutropenia (1.5%), neutropenia/neutrophil count decrease (1.5%), and infusion-related reactions (1.1%)
- Fatal adverse reactions occurred in 1.1% of patients who received MARGENZA, including viral pneumonia (0.8%) and aspiration pneumonia (0.4%)
Permanent discontinuation due to an adverse reaction occurred in 3% of patients who received MARGENZA.
- Adverse reactions that resulted in permanent discontinuation in >1% of patients who received MARGENZA included left ventricular dysfunction and infusion-related reactions
- Dosage interruptions due to an adverse reaction occurred in 11% of patients who received MARGENZA. Adverse reactions that required dosage interruption in >5% of patients who received MARGENZA included infusion-related reactions
H1 antagonist=histamine H1 receptor antagonist; H2 antagonist=histamine H2 receptor antagonist; IV=intravenous; PFS=progression-free survival.
References:
1. MARGENZA Prescribing Information. MacroGenics, Inc.; 2023.
2. Rugo HS, Im S, Cardoso F, et al. Phase 3 SOPHIA study of margetuximab + chemotherapy versus trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: Final overall survival analysis. Presented at San Antonio Breast Cancer Symposium on December 9, 2021 (#2484).
3. Data on File. Clinical Trial Protocol: CP MGAH22 04. Protocol Amendment 3.
Dosage and administration
information
Be in the know
Register for the latest information and updates about MARGENZA
Progression-free survival (PFS) results from the SOPHIA clinical trial
Prespecified exploratory PFS analysis: Subgroups by Fcγ receptor genotype1,a
CD16A-158F (FF or FV), n=437 (86%)
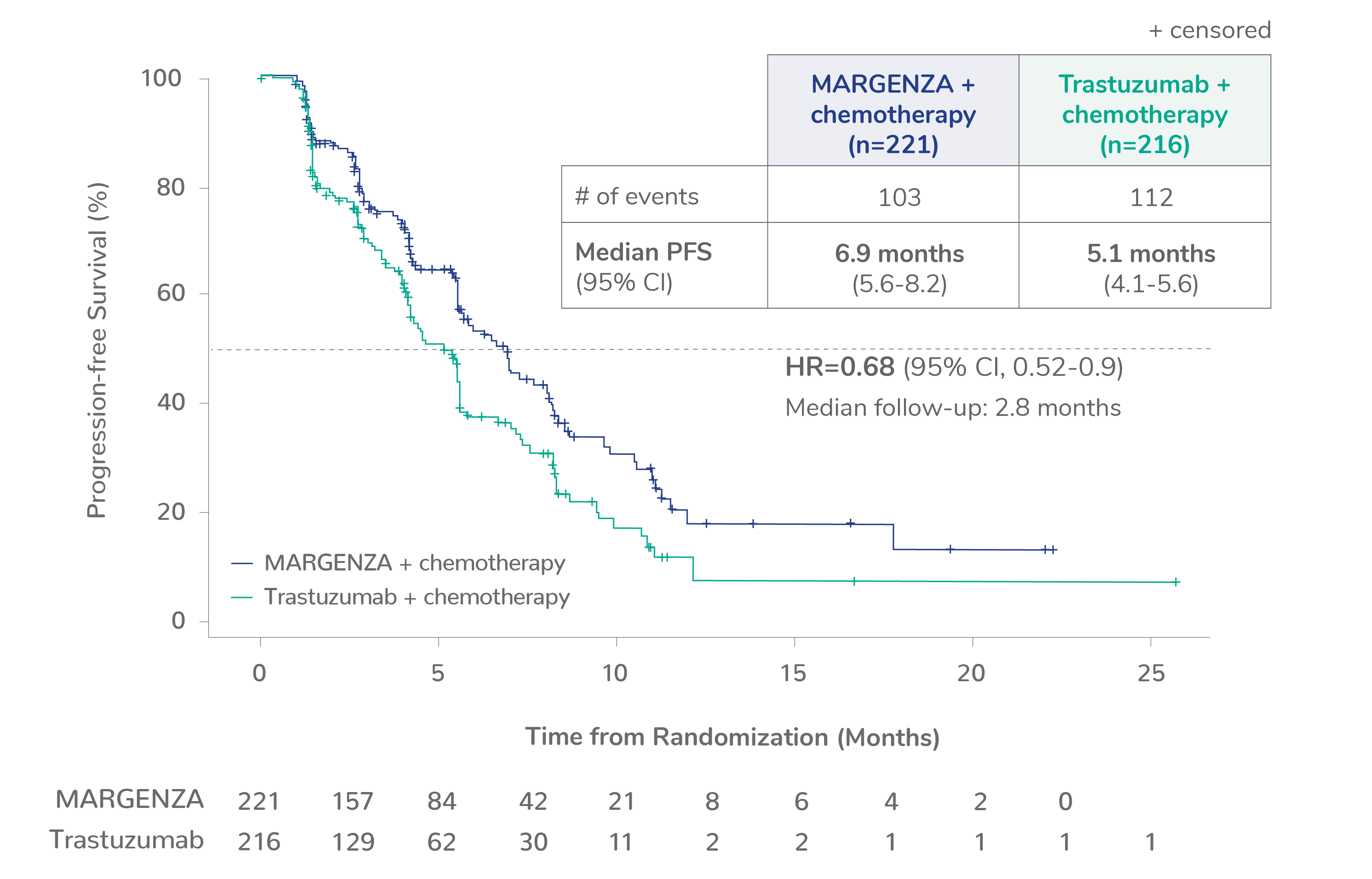
CD16A-158FF, n=192 (38%)
CD16A-158FV, n=245 (48%)
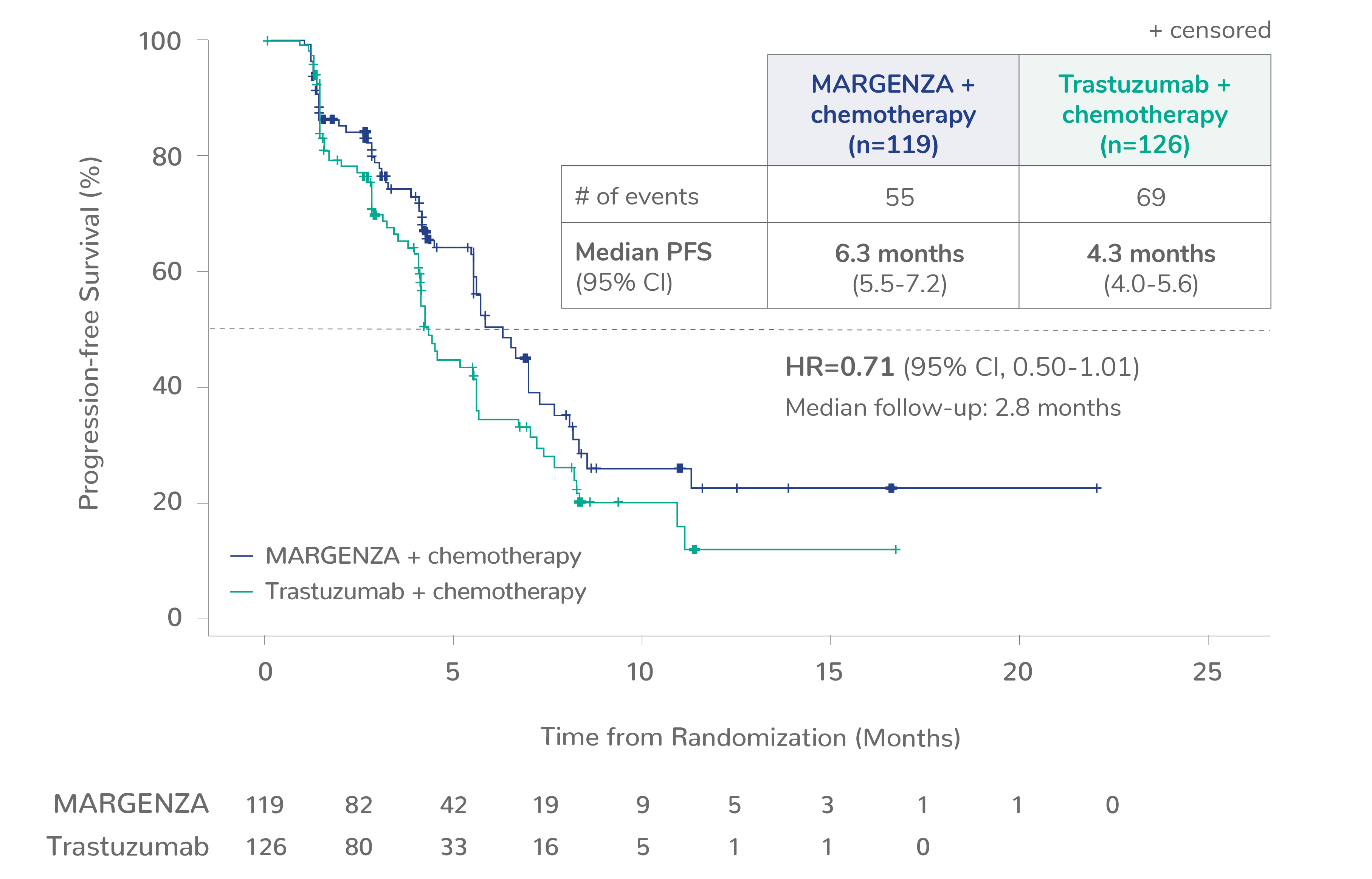
CD16A-158VV, n=69 (14%)
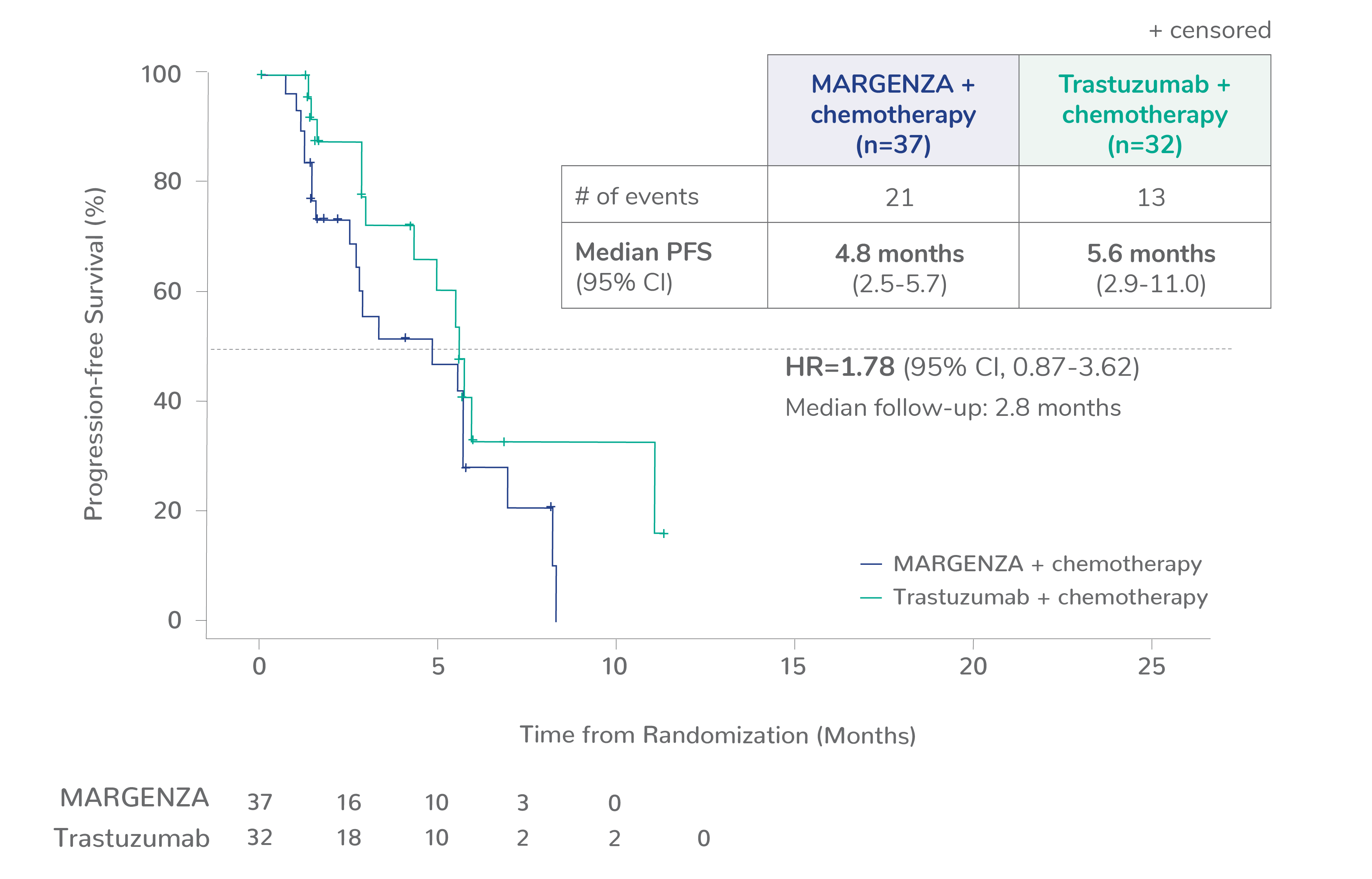
Limitation: These are exploratory analyses; therefore, the data require cautious interpretation and could represent chance findings.
a506 of 536 intention-to-treat patients were genotyped (94%)
CI=confidence interval; HR=hazard ratio
Reference:
1. Rugo HS, Im S, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial – Supplemental online content. JAMA Oncol. Published online January 22, 2021. doi: 10.1001/jamaoncol.2020.7932
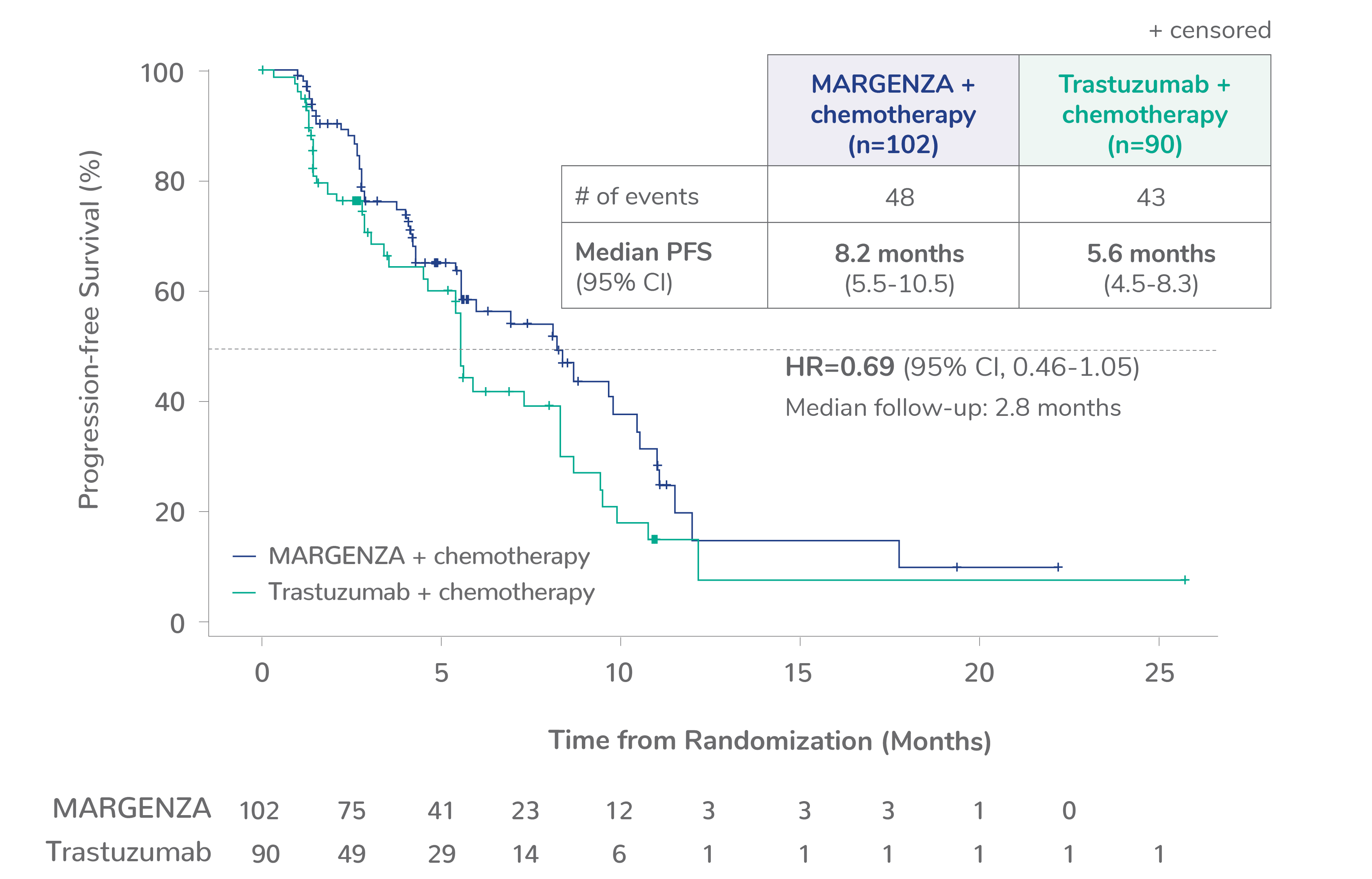
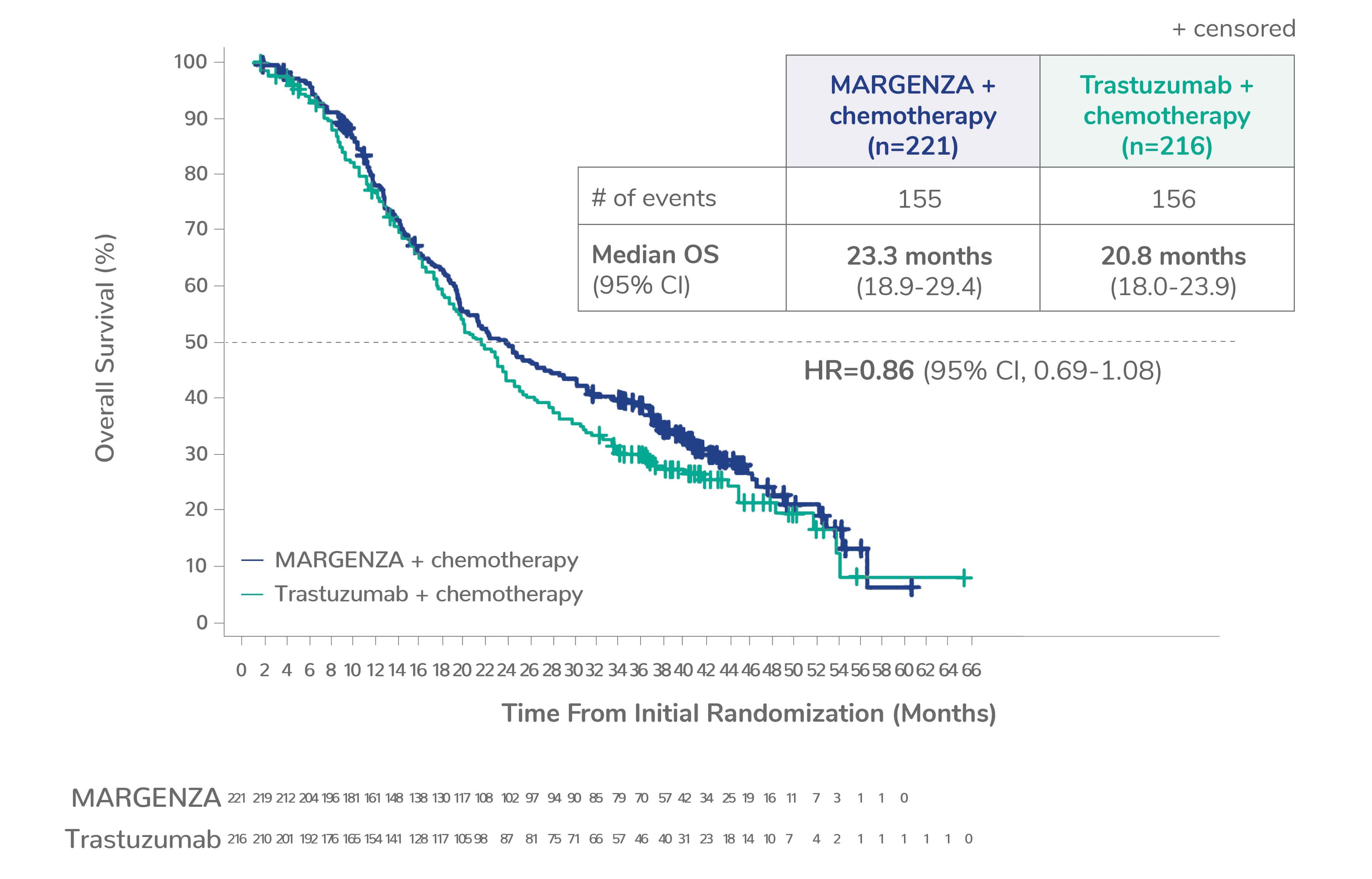
Overall survival (OS) results from the SOPHIA clinical trial
Prespecified exploratory OS analysis: Subgroups by Fcγ receptor genotype1,a
CD16A-158F (FF or FV), n=437 (86%)
CD16A-158FF, n=192 (38%)
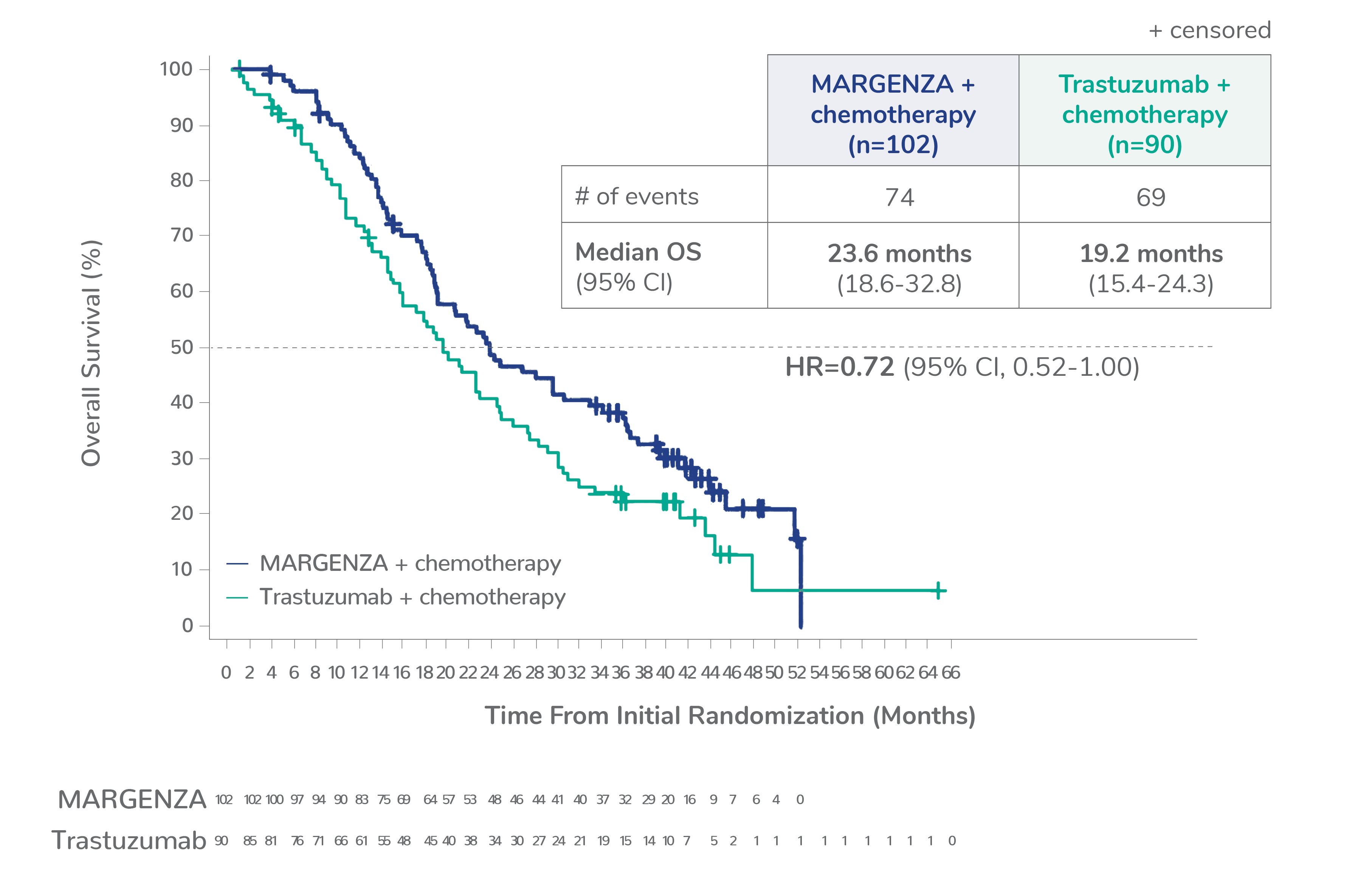
CD16A-158FV, n=245 (48%)
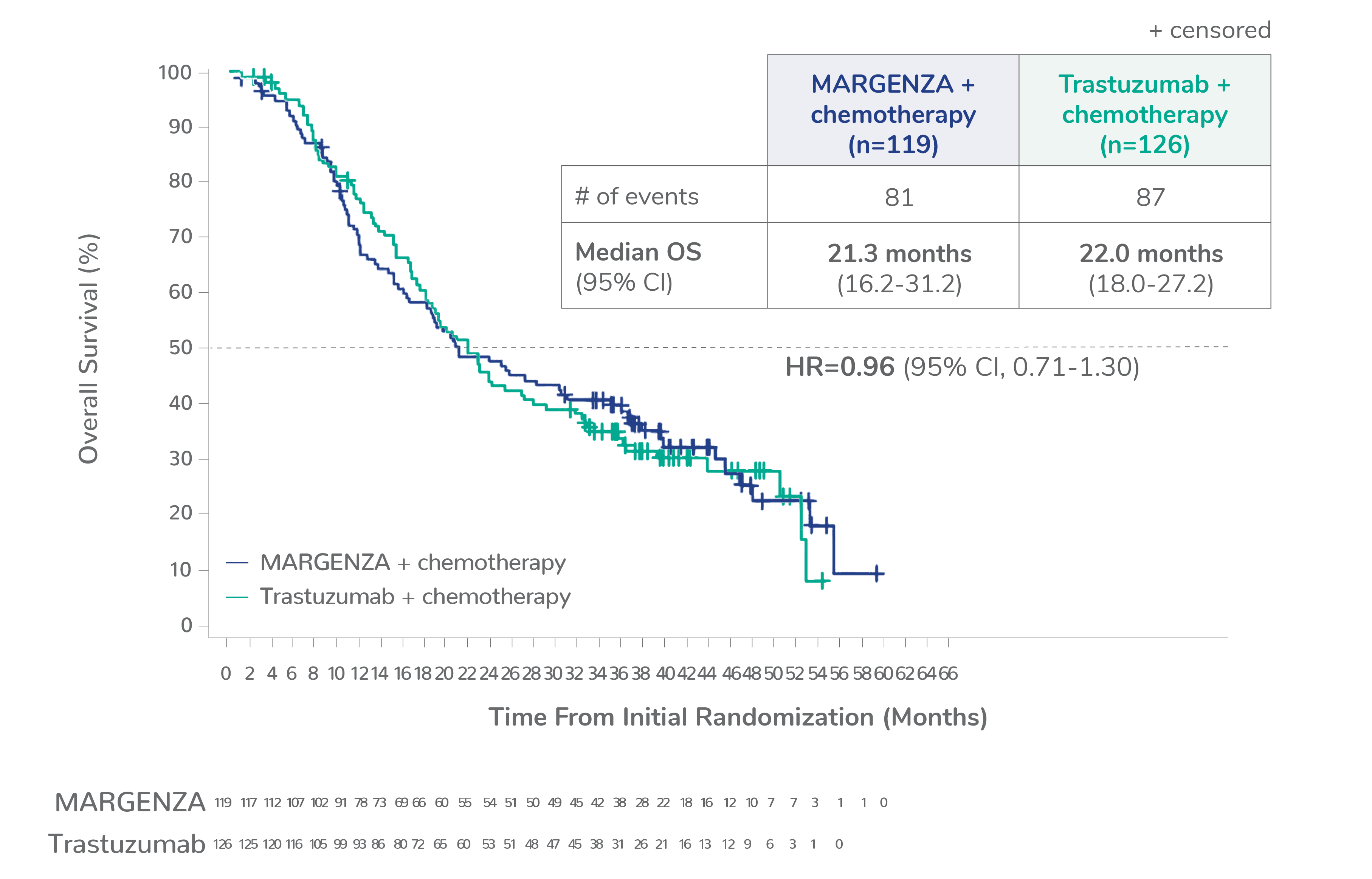
CD16A-158VV, n=69 (14%)
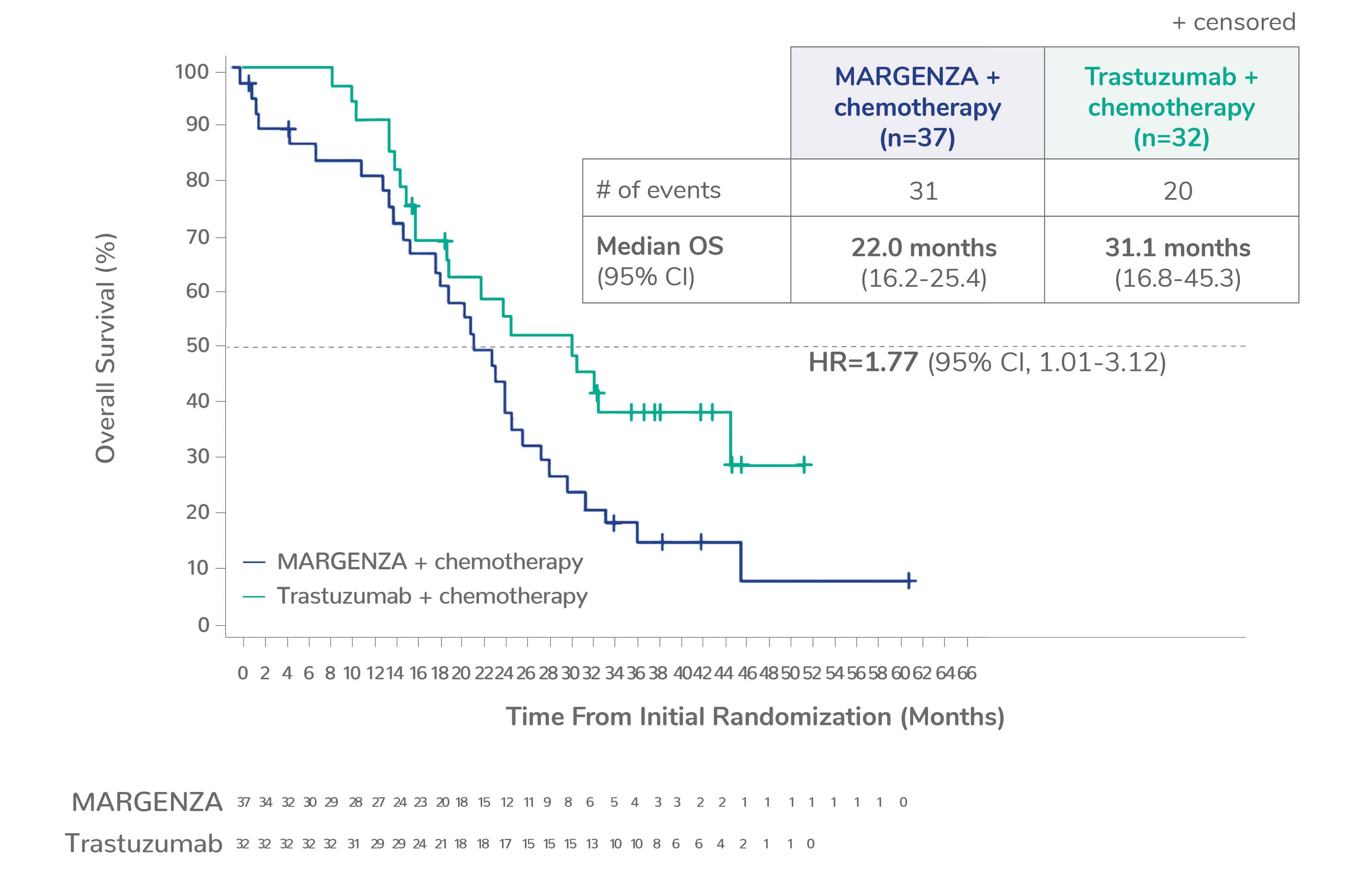
Limitation: These are exploratory analyses; therefore, the data require cautious interpretation and could represent chance findings.
a506 of 536 intention-to-treat patients were genotyped (94%)
CI=confidence interval; HR=hazard ratio
Reference:
1. Rugo HS, Im S, Cardoso F, et al. Phase 3 SOPHIA study of margetuximab + chemotherapy versus trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: Final overall survival analysis. Presented at San Antonio Breast Cancer Symposium on December 9, 2021 (#2484).
IMPORTANT SAFETY INFORMATION
WARNING: LEFT VENTRICULAR DYSFUNCTION AND EMBRYO-FETAL TOXICITY
- Left Ventricular Dysfunction: MARGENZA may lead to reductions in left ventricular ejection fraction (LVEF). Evaluate cardiac function prior to and during treatment. Discontinue MARGENZA treatment for a confirmed clinically significant decrease in left ventricular function.
- Embryo-Fetal Toxicity: Exposure to MARGENZA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception.
WARNINGS & PRECAUTIONS:
Left Ventricular Dysfunction
- Left ventricular cardiac dysfunction can occur with MARGENZA.
- In SOPHIA, left ventricular dysfunction occurred in 1.9% of patients treated with MARGENZA.
- MARGENZA has not been studied in patients with a pretreatment LVEF value of <50%, a prior history of myocardial infarction or unstable angina within 6 months, or congestive heart failure NYHA class II-IV.
- Withhold MARGENZA for ≥16% absolute decrease in LVEF from pretreatment values or LVEF below institutional limits of normal (or 50% if no limits available) and ≥10% absolute decrease in LVEF from pretreatment values.
- Permanently discontinue MARGENZA if LVEF decline persists greater than 8 weeks, or dosing is interrupted more than 3 times due to LVEF decline.
- Evaluate cardiac function within 4 weeks prior to and every 3 months during and upon completion of treatment. Conduct thorough cardiac assessment, including history, physical examination, and determination of LVEF by echocardiogram or MUGA scan.
- Monitor cardiac function every 4 weeks if MARGENZA is withheld for significant left ventricular cardiac dysfunction.
Embryo-Fetal Toxicity
- Based on findings in animals and mechanism of action, MARGENZA can cause fetal harm when administered to a pregnant woman. Post-marketing studies of other HER2 directed antibodies during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
- Verify pregnancy status of women of reproductive potential prior to initiation of MARGENZA.
- Advise pregnant women and women of reproductive potential that exposure to MARGENZA during pregnancy or within 4 months prior to conception can result in fetal harm.
- Advise women of reproductive potential to use effective contraception during treatment and for 4 months following the last dose of MARGENZA.
Infusion-Related Reactions (IRRs)
- MARGENZA can cause IRRs. Symptoms may include fever, chills, arthralgia, cough, dizziness, fatigue, nausea, vomiting, headache, diaphoresis, tachycardia, hypotension, pruritus, rash, urticaria, and dyspnea.
- In SOPHIA, IRRs were reported by 13% of patients on MARGENZA plus chemotherapy. Most of the IRRs occur during Cycle 1. Grade 3 IRRs were reported in 1.5% of MARGENZA-treated patients.
- Monitor patients during and after MARGENZA infusion. Have medications and emergency equipment to treat IRRs available for immediate use.
- In patients experiencing mild or moderate IRRs, decrease rate of infusion and consider premedications, including antihistamines, corticosteroids, and antipyretics. Monitor patients until symptoms completely resolve.
- Interrupt MARGENZA infusion in patients experiencing dyspnea or clinically significant hypotension and intervene with supportive medical therapy as needed. Permanently discontinue MARGENZA in all patients with severe or life-threatening IRRs.
MOST COMMON ADVERSE REACTIONS:
The most common adverse drug reactions (>10%) with MARGENZA in combination with chemotherapy are fatigue/asthenia (57%), nausea (33%), diarrhea (25%), vomiting (21%), constipation (19%), headache (19%), pyrexia (19%), alopecia (18%), abdominal pain (17%), peripheral neuropathy (16%), arthralgia/myalgia (14%), cough (14%), decreased appetite (14%), dyspnea (13%), infusion-related reactions (13%), palmar-plantar erythrodysesthesia (13%), and extremity pain (11%).
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch or to MacroGenics at (844)-MED-MGNX (844-633-6469).
INDICATION
MARGENZA is a HER2/neu receptor antagonist indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
Please see full Prescribing Information, including Boxed Warning.
IMPORTANT SAFETY INFORMATION
WARNING: LEFT VENTRICULAR DYSFUNCTION AND EMBRYO-FETAL TOXICITY
- Left Ventricular Dysfunction: MARGENZA may lead to reductions in left ventricular ejection fraction (LVEF). Evaluate cardiac function prior to and during treatment. Discontinue MARGENZA treatment for a confirmed clinically significant decrease in left ventricular function.
- Embryo-Fetal Toxicity: Exposure to MARGENZA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception.
WARNINGS & PRECAUTIONS:
Left Ventricular Dysfunction
- Left ventricular cardiac dysfunction can occur with MARGENZA.
- In SOPHIA, left ventricular dysfunction occurred in 1.9% of patients treated with MARGENZA.
- MARGENZA has not been studied in patients with a pretreatment LVEF value of <50%, a prior history of myocardial infarction or unstable angina within 6 months, or congestive heart failure NYHA class II-IV.
- Withhold MARGENZA for ≥16% absolute decrease in LVEF from pretreatment values or LVEF below institutional limits of normal (or 50% if no limits available) and ≥10% absolute decrease in LVEF from pretreatment values.
- Permanently discontinue MARGENZA if LVEF decline persists greater than 8 weeks, or dosing is interrupted more than 3 times due to LVEF decline.
- Evaluate cardiac function within 4 weeks prior to and every 3 months during and upon completion of treatment. Conduct thorough cardiac assessment, including history, physical examination, and determination of LVEF by echocardiogram or MUGA scan.
- Monitor cardiac function every 4 weeks if MARGENZA is withheld for significant left ventricular cardiac dysfunction.
Embryo-Fetal Toxicity
- Based on findings in animals and mechanism of action, MARGENZA can cause fetal harm when administered to a pregnant woman. Post-marketing studies of other HER2 directed antibodies during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
- Verify pregnancy status of women of reproductive potential prior to initiation of MARGENZA.
- Advise pregnant women and women of reproductive potential that exposure to MARGENZA during pregnancy or within 4 months prior to conception can result in fetal harm.
- Advise women of reproductive potential to use effective contraception during treatment and for 4 months following the last dose of MARGENZA.
Infusion-Related Reactions (IRRs)
- MARGENZA can cause IRRs. Symptoms may include fever, chills, arthralgia, cough, dizziness, fatigue, nausea, vomiting, headache, diaphoresis, tachycardia, hypotension, pruritus, rash, urticaria, and dyspnea.
- In SOPHIA, IRRs were reported by 13% of patients on MARGENZA plus chemotherapy. Most of the IRRs occur during Cycle 1. Grade 3 IRRs were reported in 1.5% of MARGENZA-treated patients.
- Monitor patients during and after MARGENZA infusion. Have medications and emergency equipment to treat IRRs available for immediate use.
- In patients experiencing mild or moderate IRRs, decrease rate of infusion and consider premedications, including antihistamines, corticosteroids, and antipyretics. Monitor patients until symptoms completely resolve.
- Interrupt MARGENZA infusion in patients experiencing dyspnea or clinically significant hypotension and intervene with supportive medical therapy as needed. Permanently discontinue MARGENZA in all patients with severe or life-threatening IRRs.
MOST COMMON ADVERSE REACTIONS:
The most common adverse drug reactions (>10%) with MARGENZA in combination with chemotherapy are fatigue/asthenia (57%), nausea (33%), diarrhea (25%), vomiting (21%), constipation (19%), headache (19%), pyrexia (19%), alopecia (18%), abdominal pain (17%), peripheral neuropathy (16%), arthralgia/myalgia (14%), cough (14%), decreased appetite (14%), dyspnea (13%), infusion-related reactions (13%), palmar-plantar erythrodysesthesia (13%), and extremity pain (11%).
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch or to MacroGenics at (844)-MED-MGNX (844-633-6469).
INDICATION
MARGENZA is a HER2/neu receptor antagonist indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
Please see full Prescribing Information, including Boxed Warning.
