When to consider MARGENZA for patients with HER2+ mBC
NCCN Guidelines®: HR‑Positive or ‑Negative and HER2‑Positivea,b
Systemic therapy regimens for recurrent unresectable (local or regional) or stage IV (M1) diseasea
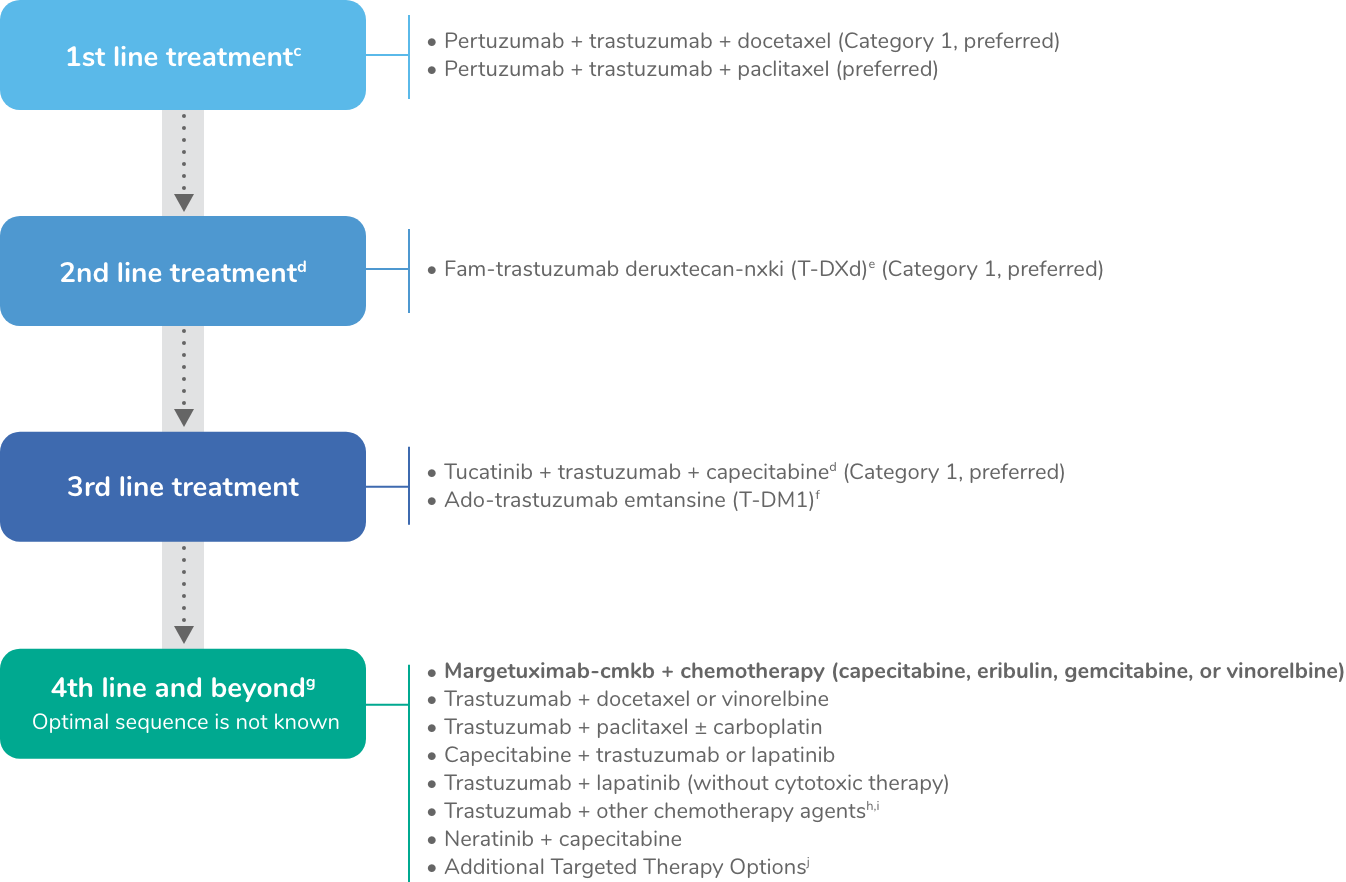
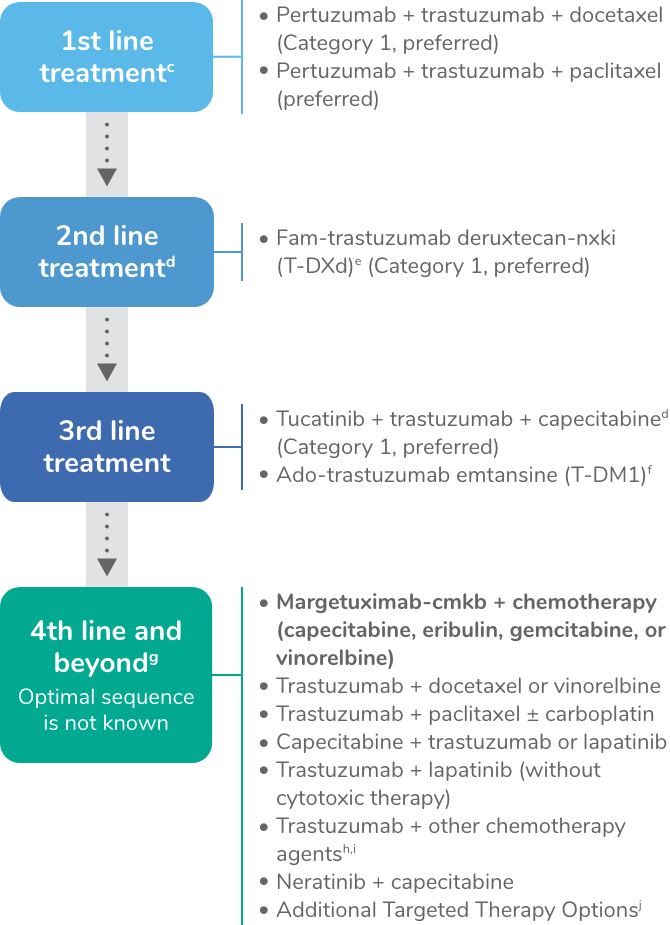
Note: All recommendations are category 2A unless otherwise indicated.
*NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
aAssess for germline BRCA1/2 mutations in all patients with recurrent or metastatic breast cancer to identify candidates for PARP inhibitor therapy. While olaparib and talazoparib are FDA-indicated in HER2-negative disease, the panel supports use in any breast cancer subtype associated with a germline mutation. There is lower-level evidence for HER2-positive tumors, therefore category 2A for this setting.
bSee additional considerations for those receiving systemic HER2-targeted therapy (BINV‑Q 4).
cMaintenance trastuzumab/pertuzumab after response (with concurrent endocrine therapy if ER+ and HER2+ metastatic breast cancer).
dTucatinib + trastuzumab + capecitabine is preferred in patients with both systemic and CNS progression in the third-line setting and beyond; and it may be given in the second-line setting.
eFam-trastuzumab deruxtecan-nxki may be considered in the first-line setting as an option for select patients (ie, those with rapid progression within 6 months of neoadjuvant or adjuvant therapy [12 months for pertuzumab-containing regimens]). Fam-trastuzumab deruxtecan-nxki is associated with interstitial lung disease (ILD)/pneumonitis. Regular monitoring for this serious side effect is recommended. For patients with a history of ILD/pneumonitis, there are no data on managing safety or toxicity of this drug in a trial.
fMay be used as an option for third-line and beyond; the optimal sequence for third-line therapy and beyond is not known. If not a candidate fam-trastuzumab T-DM1 could be considered in the second-line.
gMultiple lines of concurrent chemotherapy with anti-HER2 therapy (trastuzumab or a TKI) offer clinical benefit for recurrent unresectable HER2+ metastatic breast cancer and have been studied in phase 2 or 3 trials. Clinical experience suggests frequent clinical benefit for such treatment. However, there are no meaningful data for use of any of these regimens among patients previously treated with pertuzumab-based chemotherapy, ado-trastuzumab emtansine, fam-trastuzumab deruxtecan-nxki, or trastuzumab/capecitabine/tucatinib regimens. Thus, the optimal sequence or true benefit of therapy is not known.
hTrastuzumab given in combination with an anthracycline is associated with significant cardiac toxicity. Concurrent use of trastuzumab and pertuzumab with an anthracycline should be avoided.
iTrastuzumab may be safely combined with all non-anthracycline–containing preferred and other single agents listed on (BINV‑Q 5) for recurrent or metastatic breast cancer.
jAdditional targeted therapy options may be useful in certain circumstances when certain biomarkers are detected as listed in (BINV‑Q 6) for recurrent or metastatic breast cancer.
†After patients have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
BRCA1/2=breast cancer genes 1 and 2; CNS=central nervous system; ER+=estrogen receptor-positive; HR=hormone receptor; NCCN=National Comprehensive Cancer Network® (NCCN®); PARP=poly (ADP-ribose) polymerase; TKI=tyrosine kinase inhibitor.
Reference:
1. Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2023. © 2023 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available.
Be in the know
Register for the latest information and updates about MARGENZA
IMPORTANT SAFETY INFORMATION
WARNING: LEFT VENTRICULAR DYSFUNCTION AND EMBRYO-FETAL TOXICITY
- Left Ventricular Dysfunction: MARGENZA may lead to reductions in left ventricular ejection fraction (LVEF). Evaluate cardiac function prior to and during treatment. Discontinue MARGENZA treatment for a confirmed clinically significant decrease in left ventricular function.
- Embryo-Fetal Toxicity: Exposure to MARGENZA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception.
WARNINGS & PRECAUTIONS:
Left Ventricular Dysfunction
- Left ventricular cardiac dysfunction can occur with MARGENZA.
- In SOPHIA, left ventricular dysfunction occurred in 1.9% of patients treated with MARGENZA.
- MARGENZA has not been studied in patients with a pretreatment LVEF value of <50%, a prior history of myocardial infarction or unstable angina within 6 months, or congestive heart failure NYHA class II-IV.
- Withhold MARGENZA for ≥16% absolute decrease in LVEF from pretreatment values or LVEF below institutional limits of normal (or 50% if no limits available) and ≥10% absolute decrease in LVEF from pretreatment values.
- Permanently discontinue MARGENZA if LVEF decline persists greater than 8 weeks, or dosing is interrupted more than 3 times due to LVEF decline.
- Evaluate cardiac function within 4 weeks prior to and every 3 months during and upon completion of treatment. Conduct thorough cardiac assessment, including history, physical examination, and determination of LVEF by echocardiogram or MUGA scan.
- Monitor cardiac function every 4 weeks if MARGENZA is withheld for significant left ventricular cardiac dysfunction.
Embryo-Fetal Toxicity
- Based on findings in animals and mechanism of action, MARGENZA can cause fetal harm when administered to a pregnant woman. Post-marketing studies of other HER2 directed antibodies during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
- Verify pregnancy status of women of reproductive potential prior to initiation of MARGENZA.
- Advise pregnant women and women of reproductive potential that exposure to MARGENZA during pregnancy or within 4 months prior to conception can result in fetal harm.
- Advise women of reproductive potential to use effective contraception during treatment and for 4 months following the last dose of MARGENZA.
Infusion-Related Reactions (IRRs)
- MARGENZA can cause IRRs. Symptoms may include fever, chills, arthralgia, cough, dizziness, fatigue, nausea, vomiting, headache, diaphoresis, tachycardia, hypotension, pruritus, rash, urticaria, and dyspnea.
- In SOPHIA, IRRs were reported by 13% of patients on MARGENZA plus chemotherapy. Most of the IRRs occur during Cycle 1. Grade 3 IRRs were reported in 1.5% of MARGENZA-treated patients.
- Monitor patients during and after MARGENZA infusion. Have medications and emergency equipment to treat IRRs available for immediate use.
- In patients experiencing mild or moderate IRRs, decrease rate of infusion and consider premedications, including antihistamines, corticosteroids, and antipyretics. Monitor patients until symptoms completely resolve.
- Interrupt MARGENZA infusion in patients experiencing dyspnea or clinically significant hypotension and intervene with supportive medical therapy as needed. Permanently discontinue MARGENZA in all patients with severe or life-threatening IRRs.
MOST COMMON ADVERSE REACTIONS:
The most common adverse drug reactions (>10%) with MARGENZA in combination with chemotherapy are fatigue/asthenia (57%), nausea (33%), diarrhea (25%), vomiting (21%), constipation (19%), headache (19%), pyrexia (19%), alopecia (18%), abdominal pain (17%), peripheral neuropathy (16%), arthralgia/myalgia (14%), cough (14%), decreased appetite (14%), dyspnea (13%), infusion-related reactions (13%), palmar-plantar erythrodysesthesia (13%), and extremity pain (11%).
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch or to MacroGenics at (844)-MED-MGNX (844-633-6469).
INDICATION
MARGENZA is a HER2/neu receptor antagonist indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
Please see full Prescribing Information, including Boxed Warning.
IMPORTANT SAFETY INFORMATION
WARNING: LEFT VENTRICULAR DYSFUNCTION AND EMBRYO-FETAL TOXICITY
- Left Ventricular Dysfunction: MARGENZA may lead to reductions in left ventricular ejection fraction (LVEF). Evaluate cardiac function prior to and during treatment. Discontinue MARGENZA treatment for a confirmed clinically significant decrease in left ventricular function.
- Embryo-Fetal Toxicity: Exposure to MARGENZA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception.
WARNINGS & PRECAUTIONS:
Left Ventricular Dysfunction
- Left ventricular cardiac dysfunction can occur with MARGENZA.
- In SOPHIA, left ventricular dysfunction occurred in 1.9% of patients treated with MARGENZA.
- MARGENZA has not been studied in patients with a pretreatment LVEF value of <50%, a prior history of myocardial infarction or unstable angina within 6 months, or congestive heart failure NYHA class II-IV.
- Withhold MARGENZA for ≥16% absolute decrease in LVEF from pretreatment values or LVEF below institutional limits of normal (or 50% if no limits available) and ≥10% absolute decrease in LVEF from pretreatment values.
- Permanently discontinue MARGENZA if LVEF decline persists greater than 8 weeks, or dosing is interrupted more than 3 times due to LVEF decline.
- Evaluate cardiac function within 4 weeks prior to and every 3 months during and upon completion of treatment. Conduct thorough cardiac assessment, including history, physical examination, and determination of LVEF by echocardiogram or MUGA scan.
- Monitor cardiac function every 4 weeks if MARGENZA is withheld for significant left ventricular cardiac dysfunction.
Embryo-Fetal Toxicity
- Based on findings in animals and mechanism of action, MARGENZA can cause fetal harm when administered to a pregnant woman. Post-marketing studies of other HER2 directed antibodies during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
- Verify pregnancy status of women of reproductive potential prior to initiation of MARGENZA.
- Advise pregnant women and women of reproductive potential that exposure to MARGENZA during pregnancy or within 4 months prior to conception can result in fetal harm.
- Advise women of reproductive potential to use effective contraception during treatment and for 4 months following the last dose of MARGENZA.
Infusion-Related Reactions (IRRs)
- MARGENZA can cause IRRs. Symptoms may include fever, chills, arthralgia, cough, dizziness, fatigue, nausea, vomiting, headache, diaphoresis, tachycardia, hypotension, pruritus, rash, urticaria, and dyspnea.
- In SOPHIA, IRRs were reported by 13% of patients on MARGENZA plus chemotherapy. Most of the IRRs occur during Cycle 1. Grade 3 IRRs were reported in 1.5% of MARGENZA-treated patients.
- Monitor patients during and after MARGENZA infusion. Have medications and emergency equipment to treat IRRs available for immediate use.
- In patients experiencing mild or moderate IRRs, decrease rate of infusion and consider premedications, including antihistamines, corticosteroids, and antipyretics. Monitor patients until symptoms completely resolve.
- Interrupt MARGENZA infusion in patients experiencing dyspnea or clinically significant hypotension and intervene with supportive medical therapy as needed. Permanently discontinue MARGENZA in all patients with severe or life-threatening IRRs.
MOST COMMON ADVERSE REACTIONS:
The most common adverse drug reactions (>10%) with MARGENZA in combination with chemotherapy are fatigue/asthenia (57%), nausea (33%), diarrhea (25%), vomiting (21%), constipation (19%), headache (19%), pyrexia (19%), alopecia (18%), abdominal pain (17%), peripheral neuropathy (16%), arthralgia/myalgia (14%), cough (14%), decreased appetite (14%), dyspnea (13%), infusion-related reactions (13%), palmar-plantar erythrodysesthesia (13%), and extremity pain (11%).
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch or to MacroGenics at (844)-MED-MGNX (844-633-6469).
INDICATION
MARGENZA is a HER2/neu receptor antagonist indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
Please see full Prescribing Information, including Boxed Warning.
